
įor this reason, Schönbein is generally credited with the discovery of ozone.

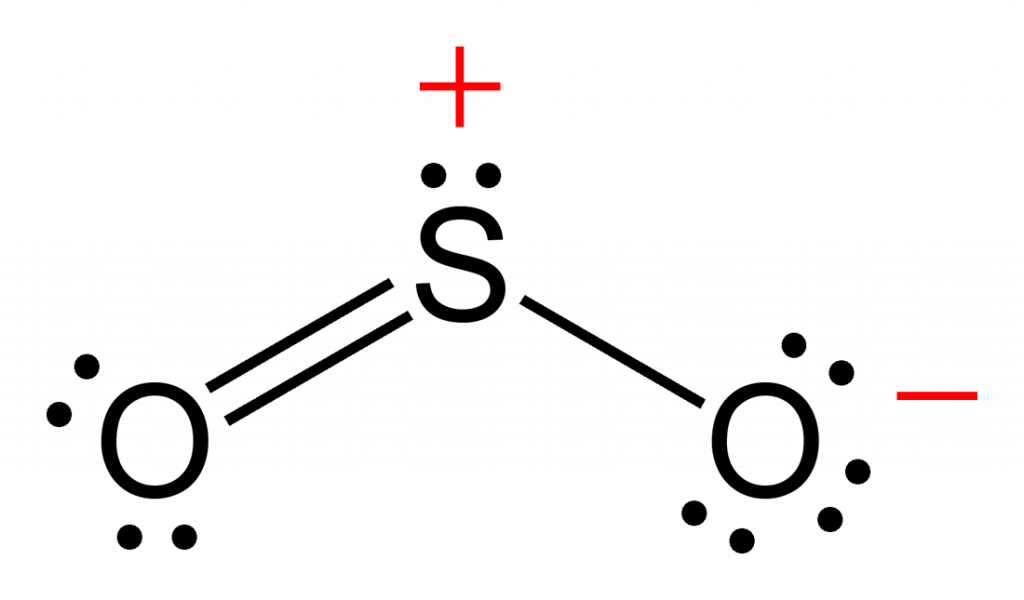
In 1839, he succeeded in isolating the gaseous chemical and named it "ozone", from the Greek word ozein ( ὄζειν) meaning "to smell". Ī half century later, Christian Friedrich Schönbein noticed the same pungent odour and recognized it as the smell often following a bolt of lightning. In 1785, Dutch chemist Martinus van Marum was conducting experiments involving electrical sparking above water when he noticed an unusual smell, which he attributed to the electrical reactions, failing to realize that he had in fact created ozone. History Christian Friedrich Schönbein (18 October 1799 – 29 August 1868) A prototype ozonometer built by John Smyth in 1865 Care should be taken to avoid confusing the name of the group for the context-specific name for the ozone given above. Trioxidanediyl (or ozonide) is used, non-systematically, to refer to the substituent group (-OOO-). In an even more specific context, this can also name the non-radical singlet ground state, whereas the diradical state is named trioxidanediyl. By default, these names pay no regard to the radicality of the ozone molecule. In appropriate contexts, ozone can be viewed as trioxidane with two hydrogen atoms removed, and as such, trioxidanylidene may be used as a systematic name, according to substitutive nomenclature. The name ozone derives from ozein (ὄζειν), the Greek neuter present participle for smell, referring to ozone's distinctive smell. The systematic names 2λ 4-trioxidiene and catena-trioxygen, valid IUPAC names, are constructed according to the substitutive and additive nomenclatures, respectively. The trivial name ozone is the most commonly used and preferred IUPAC name. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface. This same high oxidizing potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. Ozone is a powerful oxidant (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. It is therefore used commercially only in low concentrations. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures, physical shock, or fast warming to the boiling point. In standard conditions, ozone is a pale blue gas that condenses at cryogenic temperatures to a dark blue liquid and finally a violet-black solid. The molecule was later proven to have a bent structure and to be weakly diamagnetic. Ozone's O 3 structure was determined in 1865. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as 0.1 ppm in air. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is an allotrope of oxygen that is much less stable than the diatomic allotrope OĢ, breaking down in the lower atmosphere to OĢ ( dioxygen). It is a pale blue gas with a distinctively pungent smell. Ozone ( / ˈ oʊ z oʊ n/) (or trioxygen) is an inorganic molecule with the chemical formula Oģ.


 0 kommentar(er)
0 kommentar(er)
